- Service Scope
- Medical Device Registration and File Clinical Evaluation Legal Agent Medical Device QMS Supporting Regulatory Consulting
- RS Originals
- Useful Infromation Original Articals
- Contact Us

Follow our WeChat to stay up-to-date
on NMPA regulation


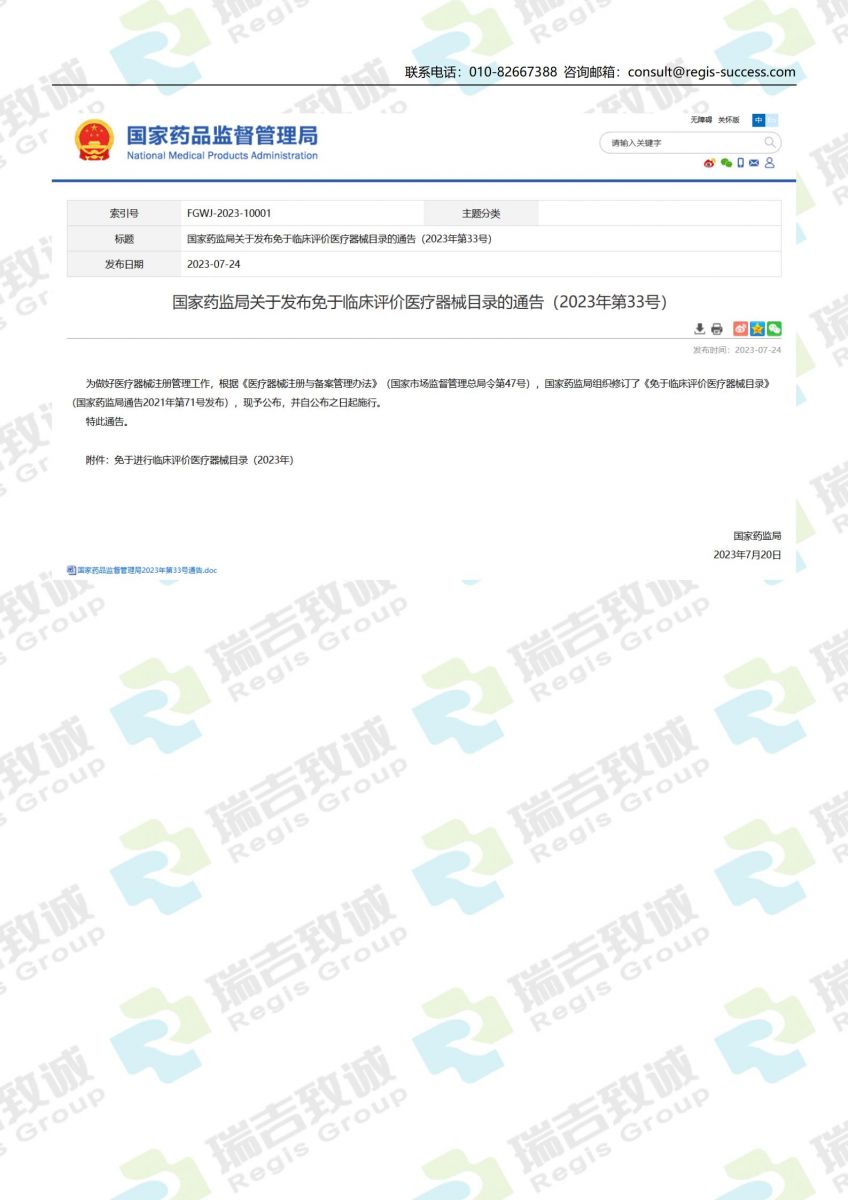
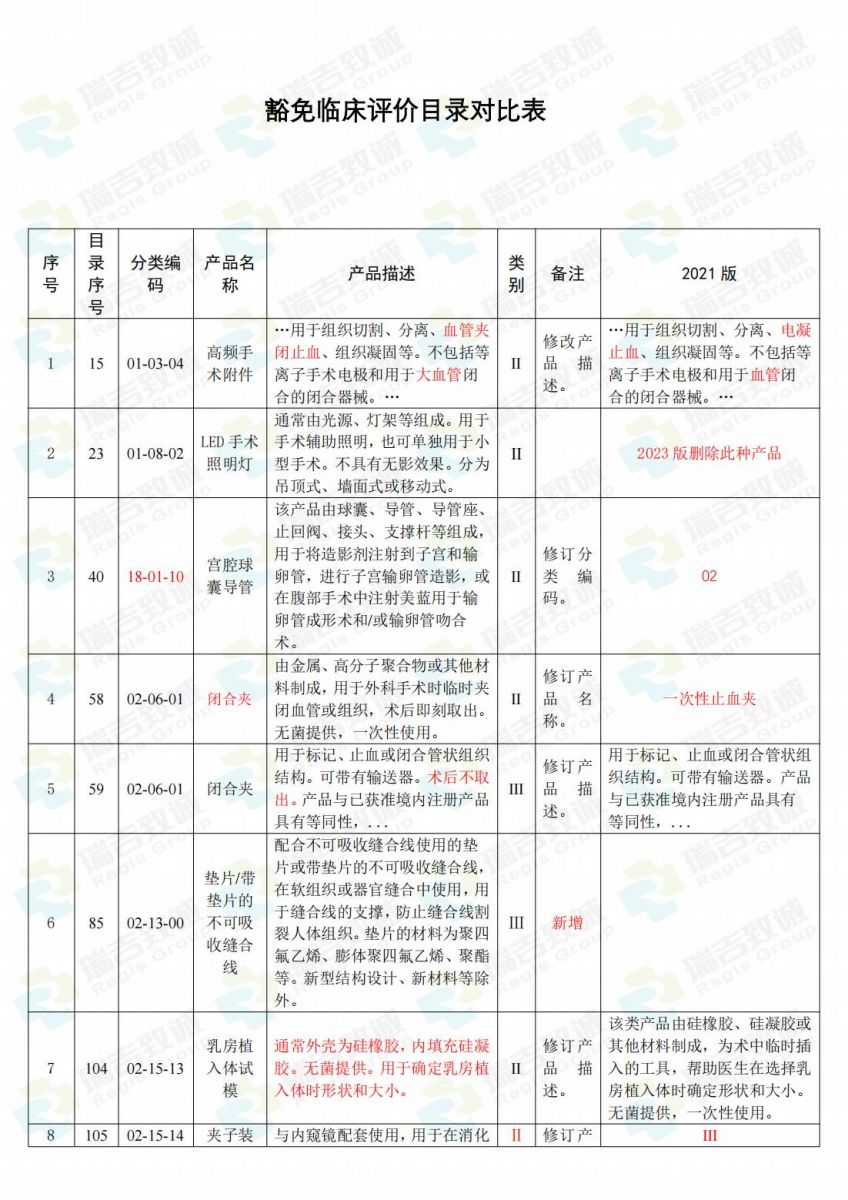
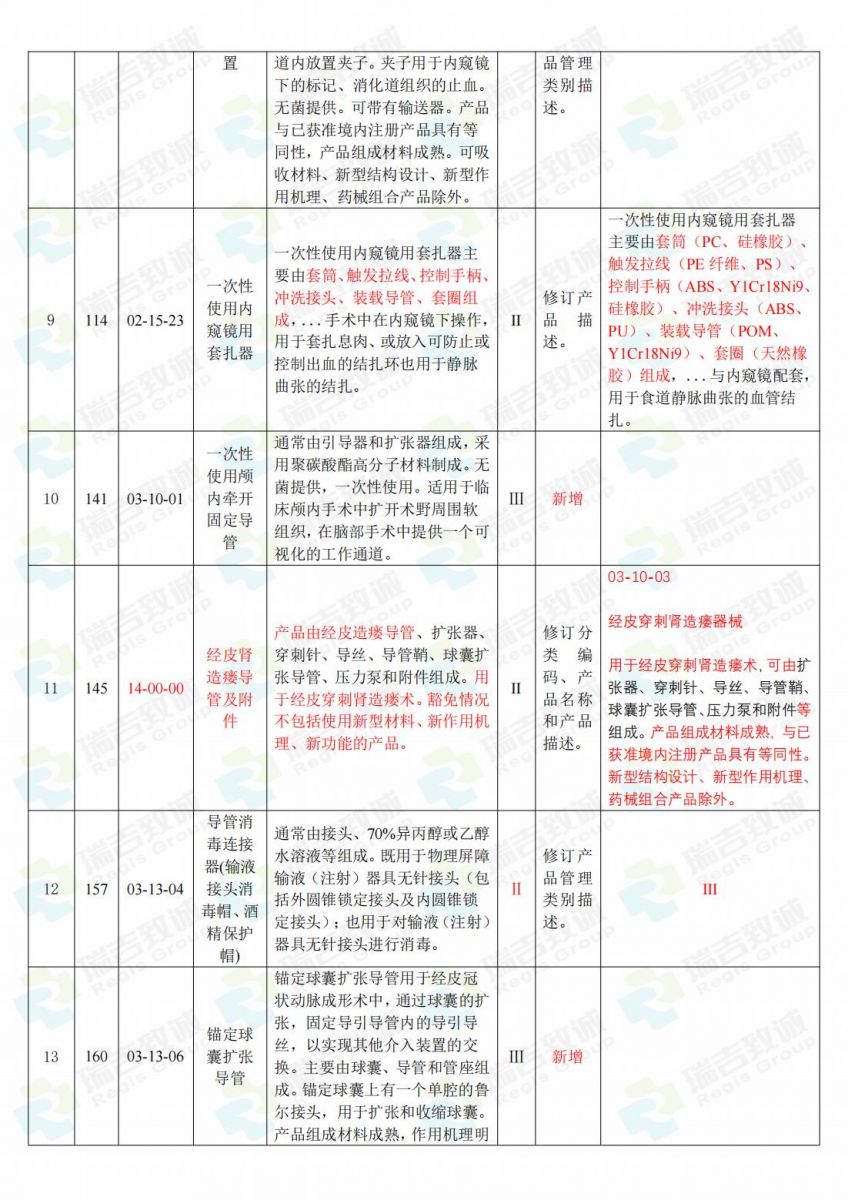
.jpg)
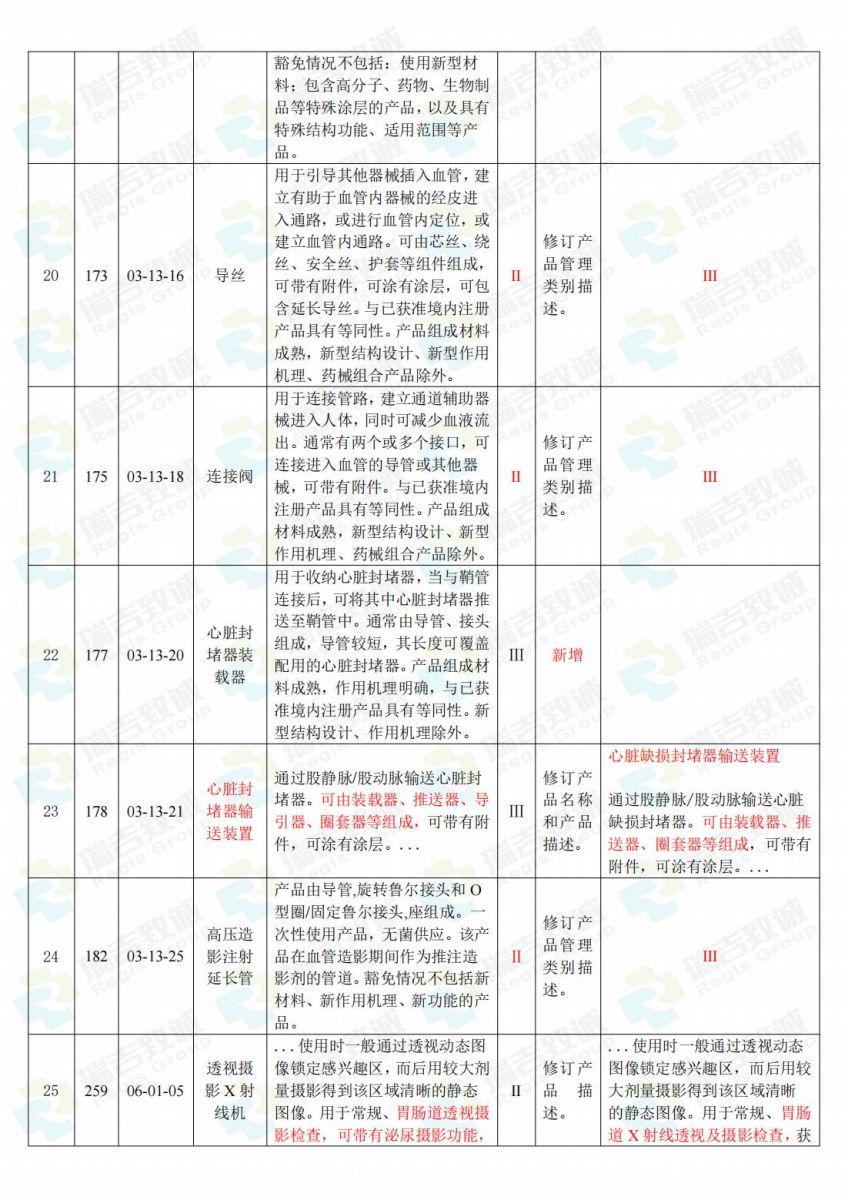
.jpg)
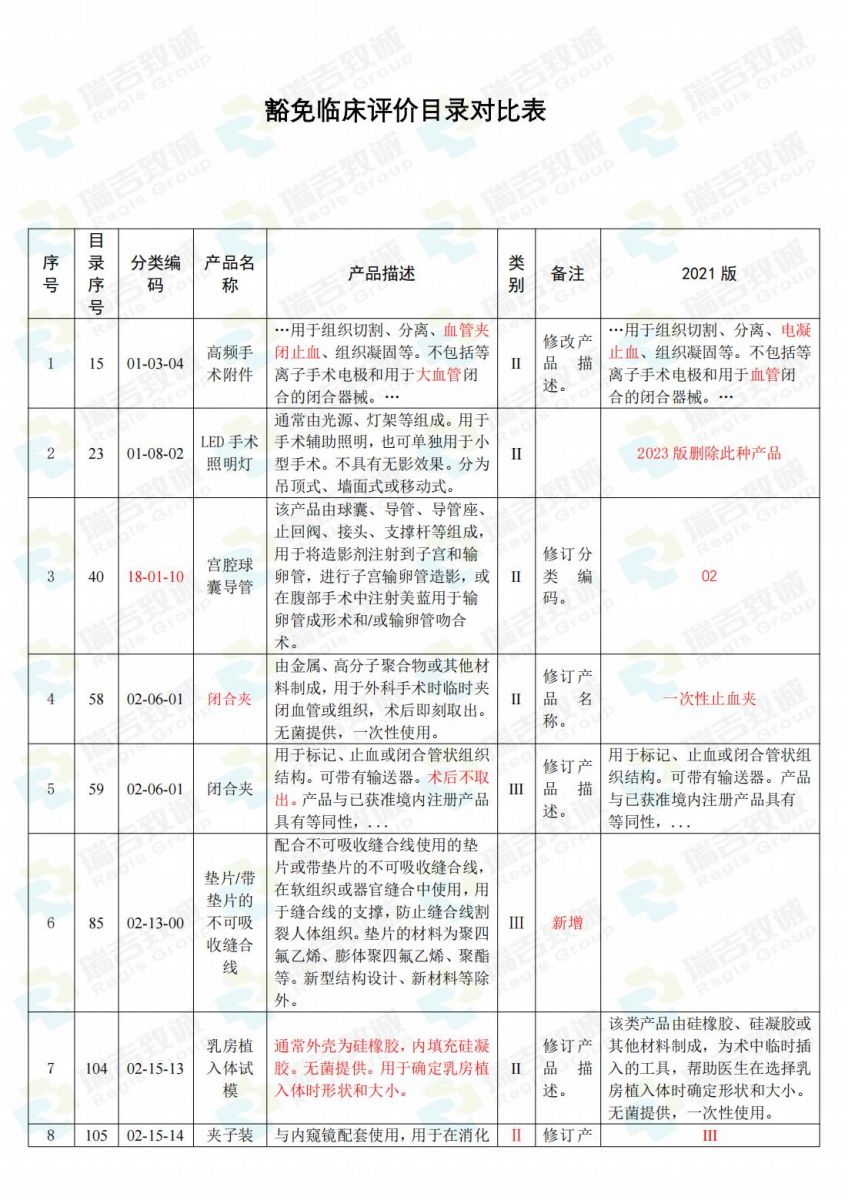
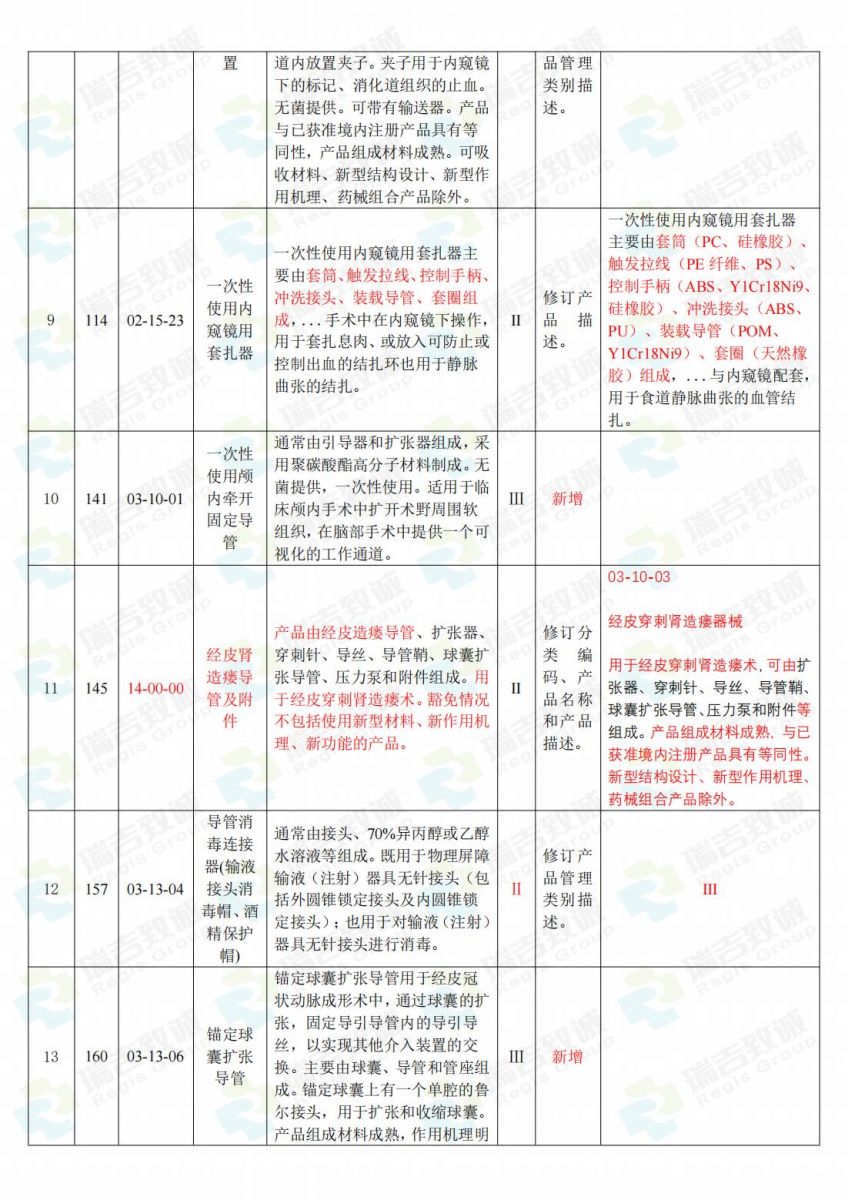
.jpg)
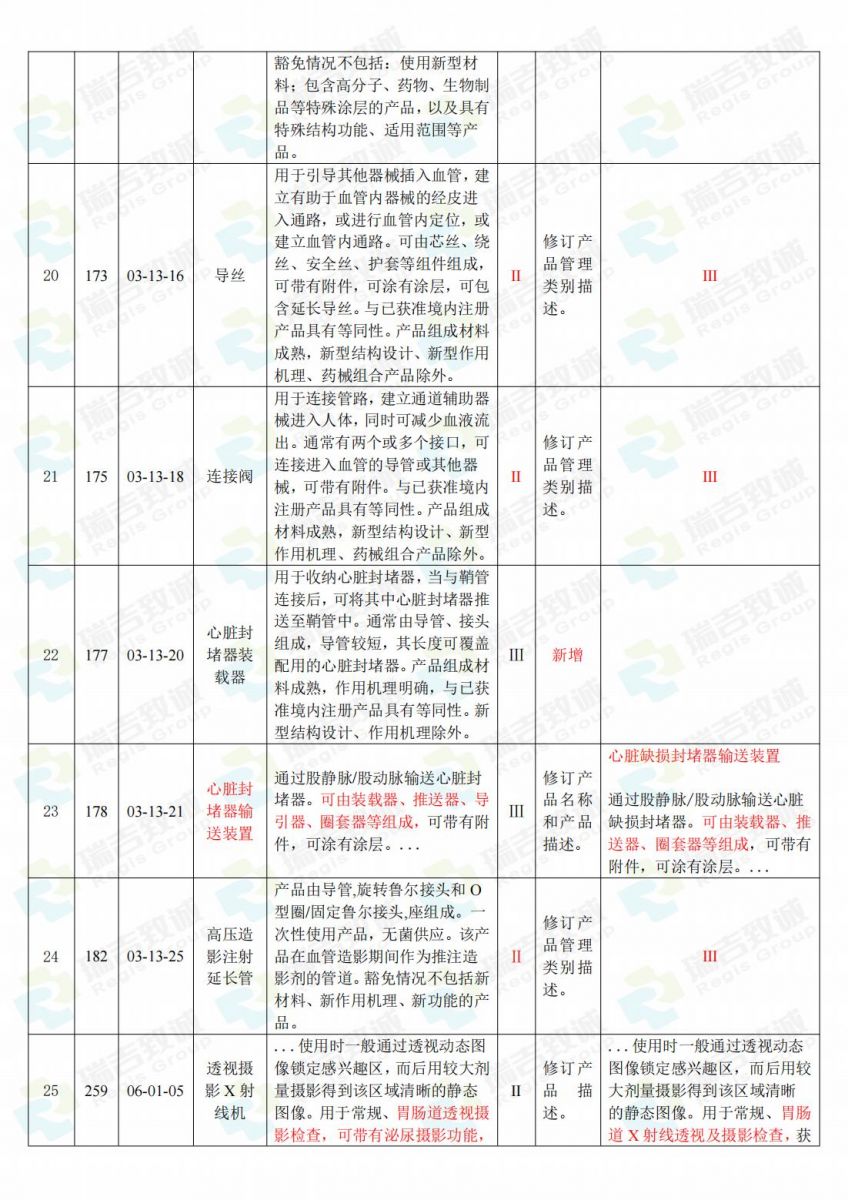
.jpg)

